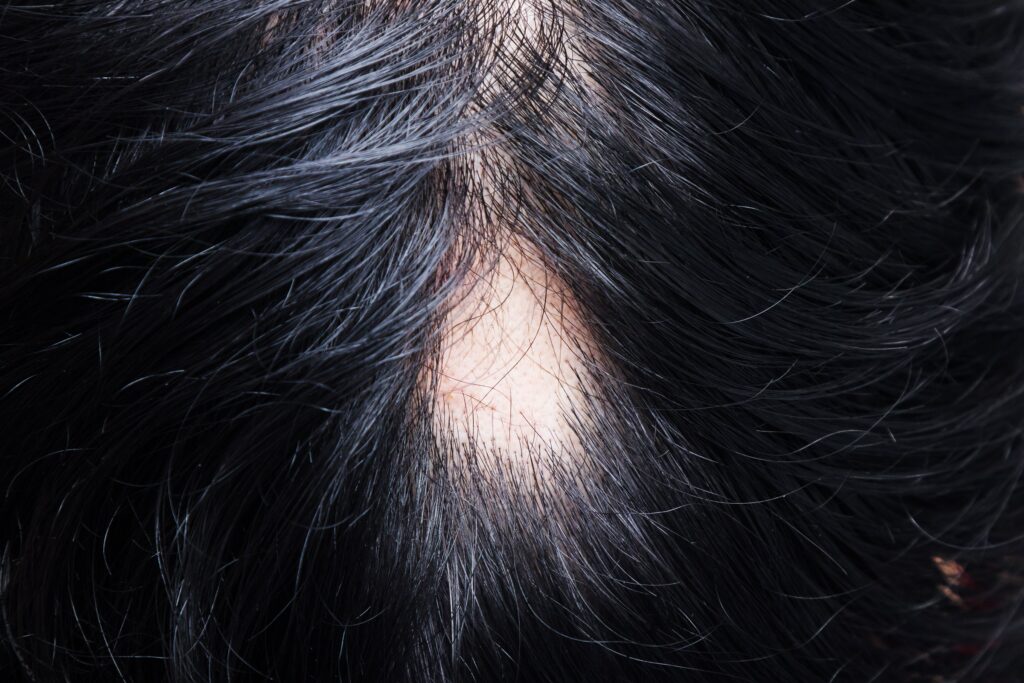
Denise Jones quit her job because she was losing her hair. Diagnosed with the autoimmune disease alopecia areata , she saw her hair falling out in patches, bald spots dotting her scalp. The snarky comments and backhanded compliments had already started at her workplace, and she wasn’t sure how much more she could take.
So she left. According to her physician, Luis Garza, a professor of dermatology at Johns Hopkins University School of Medicine in the US, Denise (whose name has been changed to protect her privacy) is far from alone in experiencing stress and anxiety over hair loss. Hair, he explains, is a fundamental aspect of identity, deeply intertwined with our body image and sense of self.
That’s why baldness can, quite literally, change a person’s life. Yet despite more than 50 percent of women and 85 percent of men in the US experiencing balding during their lives, there still aren’t effective treatments for hair loss. “None of them work really well,” Garza says.
For common baldness, the two drugs approved by the US Food and Drug Administration (FDA)—finasteride and minoxidil—promote hair growth only slightly, must be used daily, and can cause side effects like depression and decreased libido . Another popular option is hair transplantation, where hair follicles are moved from one part of the scalp to another. But the procedure is invasive, expensive (costing $4,000 to $15,000 out of pocket in the US), and limited by how much hair can be moved.
Given these lackluster options, most people can’t do anything meaningful about their hair loss. But that may soon change. In a study published in Developmental Cell last month, Maksim Plikus, professor of developmental and cell biology at the University of California, Irvine, and chief scientific officer of hair biotech company Amplifica, uncovered the role of a potent signaling molecule called SCUBE3.
This protein might reshape how physicians approach baldness. With its roughly half a million hair follicles, you can think of your scalp as a gigafactory of 3D printers. According to Plikus, nearly all these follicles need to be constantly “printing” in order to create a full mop of hair.
But in common baldness, these printers start shutting down, leading to hair thinning (if roughly 50 percent have switched off) and balding (when more than 70 percent are off). By activating stem cells present in people’s scalps, SCUBE3 hacks hair follicles to restart the production line and promote rapid growth. Plikus’ research began because he wanted to better understand dermal papilla cells, which are located at the bottom of hair follicles.
It’s notoriously difficult to experiment with them, so to learn more about how they work, his team used a genetic tool to target a signaling pathway (a series of molecular reactions that involve certain proteins) that these cells use to drive hair growth. The goal was to get this pathway, in a set of hairless mice, to stay always switched on. Once he and his team got the tool to work, the genetically modified mice started rapidly growing hair.
But Plikus and his team didn’t know what exactly in this pathway was driving growth, so using single-cell RNA sequencing—a technique that lets you see what genes are active in a cell, and thus what proteins are being created—they compared cells from the genetically modified mice and control mice. They found that SCUBE3 was being expressed in the mutant mice but not in the controls. That didn’t mean anything on its own, however, because SCUBE3 could’ve just been a bystander molecule.
So they performed a series of experiments with this protein, first deleting it from mice, then injecting it into normal mice, and then injecting it into mice with human hair follicles grafted to their skins. These all showed that SCUBE3 drives hair growth and, crucially with the last experiment, human hair growth. While Plikus recognizes that much work is needed to go from mouse models to an FDA-approved treatment, he’s already envisioning a future in which patients might go to their dermatologist to get SCUBE3 microinjected into their scalps.
“You have a patient sitting in a dentist-like chair, they close their eyes, and then you go tch, tch, tch, tch ,” Plikus says as he mimics a syringe being pressed into the patient’s head. SCUBE3 would be dispensed less than a millimeter deep, with only micrograms needed, so the procedure would be short (under 20 minutes) and fairly painless, he predicts. The cost might be similar to Botox, so not cheap, but certainly less expensive than a hair transplant.
In addition, the therapy would probably need to be repeated two or three times a year to ensure continued hair growth. “Pharma would love the model,” Plikus says, because booster therapy is an attractive mix of real efficacy and repeat customers; the popularity of Botox and dermal fillers demonstrates this well. If things take off, SCUBE3 would also be easy to scale, given that culturing proteins is cheap and already widely done, as it is for insulin.
“I think it’s a realistic vision,” says Maria Kasper, associate professor of cell and molecular biology at the Karolinska Institute in Sweden. However, she emphasizes that it’s too early to say whether Plikus’ findings will lead to a new treatment for hair loss and notes that alternative therapeutic approaches are being developed as well. Turn Biotechnologies, for instance, is developing a treatment that uses messenger RNA (mRNA), following the same basic principles as the Pfizer and Moderna Covid vaccines—delivering genetic instructions to our cells to have them build useful substances.
According to cofounder Vittorio Sebastiano, an associate professor of obstetrics and gynecology at Stanford University in the US, Turn’s goal is to deliver mRNA encoding for a cocktail of proteins that can turn back the clock on hair follicles. Their treatment, TRN-001, would be delivered to follicles inside liquid nanoparticles and help reset stem cells there, making the follicles functionally younger. “I would be happy to get my hair back to when I was 30,” Sebastiano jokes, “so that would be 15 years of rejuvenation.
” Sebastiano is hoping to start clinical trials in humans by the end of next year or early 2024, envisioning a future in which TRN-001 is applied topically with microinjections, much like Plikus imagines for SCUBE3. But while an mRNA-based approach might be more potent, since it forces cells to make relevant proteins themselves, Sebastiano recognizes that this technology’s newness makes the cost and periodicity of treatment difficult to predict and the regulatory landscape more challenging. In fact, Kevin McElwee, associate professor of dermatology at the University of British Columbia in Canada and chief scientific officer of hair biotech company RepliCel, says that’s why his team isn’t going down the mRNA route: “the regulatory issues with the FDA are huge.
” Instead, RepliCel—and a competitor, HairClone—are working on a cell-based approach to baldness, where hair cells from one part of the scalp are moved to another in order to kickstart growth. First, hair follicles are harvested from the back of a person’s scalp, then the relevant cells (dermal papilla cells for HairClone, dermal sheath cup cells for RepliCel) are dissected out and cultured, and finally these multiplied cells are microinjected into a person’s balding head. Some of these cells are also cryopreserved for future injections.
“The problem with hair transplantation is that it’s one for one; you still have the same number of hairs, just spread out,” says HairClone CEO Paul Kemp. With these multiplying techniques, you can instead increase the volume of hair. However, Kemp and McElwee both estimate that for the patient, the process might take one to two months from start to finish and, at least initially, cost more than hair transplants, given the manual labor involved.
But this treatment might also be more successful, Kemp says, because “it’s a personalized cell therapy, unlike Plikus’ approach, which is a one-size-fits-all. ” RepliCel’s therapy has begun to be tested in patients in Japan, while HairClone hopes to start human trials in the UK by early 2023; both countries have more flexible clinical trial requirements than the US. Nonetheless, whether it’s with molecular, RNA, or cell-based approaches, new hair-loss treatments are coming soon.
It’s just impossible to know when. “Despite decades of trying, it’s always that the next therapy for hair loss is five years away,” Garza jokes. The problem is the “valley of death” between preclinical studies and commercialization, where hair biotech companies have long crashed and burned, he says, because baldness is so poorly understood—to this day.
“They’re trying to build skyscrapers in a swamp. ” Kasper emphasizes the need for basic scientific research to establish a stronger foundation. Her lab at the Karolinska Institute, for instance, studies how to make new hair follicles inside skin—from scratch—which is an admittedly more challenging question than how to hack existing follicles.
Beyond offering opportunities to better understand hair biology, this research emphasizes the complexity of hair loss: SCUBE3, TRN-001, and cloned cells can’t help patients who don’t have hair follicles in the first place. The only way to help such patients, who may have burns, large wounds, or scarring alopecia , is with new follicles. In all likelihood, none of these are going to be a magic bullet.
Instead, the future is probably one of multiple treatments used together, each with complementary strengths and limitations. But Garza would be happy with even just one, because in the therapeutic black hole of baldness, his patients are becoming increasingly desperate and helpless. “The state of art is terrible right now,” he says.
.
From: wired
URL: https://www.wired.com/story/new-baldness-treatments/