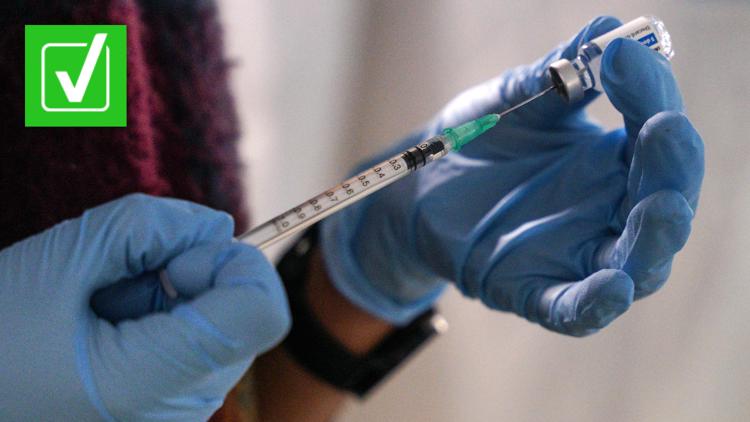
Editor’s note: This story has been updated with the latest information after the FDA authorized omicron booster shots from Pfizer and Moderna on Aug. 31. The BA.
5 subvariant of omicron, which remains dominant in the U. S. right now, is more resistant to antibody protection from vaccines and prior infection than earlier omicron strains.
That’s left some people wondering when they might be able to get another COVID-19 vaccine that targets omicron and its subvariants, Google Trends data show. A VERIFY reader also asked the team when vaccines for the new COVID-19 variants will be available. Will COVID-19 vaccine boosters for omicron be available to the public in 2022? Yes, COVID-19 vaccine boosters for omicron will be available to the public in 2022.
The FDA authorized omicron boosters from Pfizer and Moderna on Aug. 31 and vaccinations could begin within days. On Aug.
31, 2022, the U. S. Food and Drug Administration (FDA) authorized COVID-19 boosters shots with an omicron component from Pfizer and Moderna.
The bivalent vaccines, also referred to by the FDA as “updated boosters,” contain one messenger mRNA component of the original strain of the virus that causes COVID-19 and another one in common with the BA. 4 and BA. 5 omicron subvariants.
They will be given at least two months after primary or booster vaccinations. Pfizer’s omicron vaccine is authorized for use as a single booster dose in people 12 and older, while Moderna’s shot is authorized for those 18 and older, according to the FDA. Omicron boosters now await approval from the Centers for Disease Control and Prevention (CDC).
The public health agency will discuss whether to recommend the shots at a meeting of its Advisory Committee on Immunization Practices (ACIP) on Thursday, Sept. 1. The Associated Press reports that vaccinations with the updated boosters could begin within days.
The FDA has not advised manufacturers to change the vaccine for primary vaccination, since the authorized and approved COVID-19 vaccines provide a “base of protection against serious outcomes of COVID-19” caused by circulating variants. Prior to the FDA authorization of omicron boosters, the U. S.
Department of Health and Human Services (HHS) said omicron boosters purchased by the federal government may be used in the fall or winter of 2022. The FDA previously told VERIFY that the updated boosters could be available as early as September. Moderna announced on July 29 that the U.
S. government purchased 66 million doses of its updated COVID-19 vaccine booster candidate, which contains the original Spikevax vaccine and mRNA from the omicron BA. 4/BA.
5 strain. The company is also advancing another booster shot option that contains the BA. 1 omicron strain.
Several sublineages of omicron, including BA. 1, BA. 4 and BA.
5, share some of the same mutations, according to the World Health Organization (WHO) . According to the Department of Health and Human Services (HHS), the U. S.
government has also purchased 105 million COVID-19 vaccine boosters from Pfizer. Those boosters include those adapted for omicron, Pfizer said on June 29 . Novavax’s COVID-19 vaccine is not yet FDA authorized as a booster shot, but the company does have an omicron shot in the works.
In late May, Novavax announced that it would begin phase 3 of another trial to determine the effectiveness of its omicron strain vaccine as a booster dose in producing better immune responses to the omicron variant compared to its newly authorized vaccine without a booster. The trial participants include people who have received either a two-dose primary series or three-dose booster series of an mRNA vaccine. More from VERIFY : No, the Novavax COVID-19 vaccine isn’t authorized as a booster shot yet Adults who haven’t received their first booster dose, especially those who are at high risk of severe disease, should receive one soon since COVID-19 cases are rising, and not wait for an updated booster, the FDA says.
Once boosters with an omicron component are available, people should consider getting one at an appropriate time following their prior booster shot. Those who are 50 and older and haven’t received a second booster shot can still do so and receive an updated booster in the fall, according to the FDA. “Receiving a booster now will not prevent individuals from receiving an updated booster following an appropriate interval between doses once the FDA reviews the variant-specific vaccines and authorizes them for use,” the spokesperson said.
More from VERIFY : Yes, it’s safe to get the COVID-19 vaccine booster if you have mild cold symptoms The VERIFY team works to separate fact from fiction so that you can understand what is true and false. Please consider subscribing to our daily newsletter , text alerts and our YouTube channel . You can also follow us on Snapchat , Twitter , Instagram , Facebook and TikTok .
Learn More » Text: 202-410-8808.
From: wfaa
URL: https://www.wfaa.com/article/news/verify/vaccines-verify/when-omicron-covid-vaccine-boosters-will-be-available-2022/536-ff9f05f3-0a2d-4163-99c0-e44b324fdbd6