January 17, 2024 This article has been reviewed according to Science X’s editorial process and policies . Editors have highlighted the following attributes while ensuring the content’s credibility: fact-checked peer-reviewed publication trusted source proofread by Max Planck Society Catalysis is one of the key technologies in the chemical industry and has a wide-reaching impact on various aspects of our daily lives, including plastics manufacturing, drug synthesis, and production of both fertilizers and fuels. It is estimated that over 90% of chemical products are nowadays manufactured with the involvement of catalysis in at least one stage.
Catalysis is a complex process that relies on the precise structural control of several elements at the crossroads of phase (in-)stabilities. While long-term stable catalysts are indispensable in order to promote high-performance and efficient reactions, reactants undergo major chemical changes, leading to the formation of final and desired products. In heterogeneous catalysis, catalyst and reactants exist in different phases.
Among the various heterogeneous catalytic processes, dry reforming of methane (DRM) has recently become the subject of academic attention, as it converts two greenhouse gases, methane (CH 4 ) and carbon dioxide (CO 2 ), into hydrogen (H 2 ) and carbon monoxide (CO). This mixture is also known as syngas, and can be used to reduce the reliance on fossil fuels by consecutively building up larger hydrocarbons via the Fischer-Tropsch chemistry. Although nickel- and cobalt-based catalysts, being low cost and highly available on Earth, have shown promising activity for DRM, designing high-performance catalysts is often challenging, as the connection between chemical dynamics, the formation of the active surface species and their reaction pathways is generally missing.
This knowledge can only be gained from so called operando experiments in which structure and function are probed simultaneously. A collaborative effort by scientists from the Departments of Inorganic Chemistry and Theory at the Fritz Haber Institute of the Max Planck Society in Berlin has provided fundamental insights into the processes occurring at the catalyst surface and how this modulates the catalytic performance during DRM. The study is published in the journal Nature Catalysis .
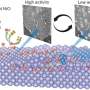
In particular, the team studied the role of different oxygen species on a nickel catalyst during DRM by using a combination of experimental and computational science techniques, including operando scanning electron microscopy, near ambient pressure X-ray photoelectron spectroscopy, and computer vision. They highlighted the critical role of dissociative CO 2 adsorption in regulating the oxygen content of the catalyst and CH 4 activation. Furthermore, they discovered the presence of three metastable oxygen species at the catalyst: atomic surface oxygen, subsurface oxygen, and bulk NiO x .
Interestingly, these exhibited different catalytic properties, and their interplay and transformation gave rise to oscillations in the surface states and in the catalytic function. They observed that some of the surface oxygen leaked into the catalyst bulk, reducing the availability of the catalyst for CH 4 activation and favoring CO 2 and O diffusion instead. The extent of the leakage was further proved by X-ray spectroscopy and transmission electron microscopy , revealing the presence of oxygen several nanometers below the surface of the catalysts.
Consequently, new metallic sites were exposed, thus leading to an increased rate of oxygen uptake and a decrease in the H 2 /CO product ratio. Lastly, they understood that co-feeding of CO 2 is essential for CH 4 conversion, likely assisting its activation together with the presence of oxygenated species. “It was impressive to see how the metastability of the Ni-O system self-adjusts the catalytic performance and that one element from the reactants can direct the entire process, which depends on its location and its chemistry.
We hope that our findings can give new momentum in adjusting longevity and selectivity in catalysis,” says PD Dr. Thomas Lunkenbein, leader of the project and co-author of the study. Understanding the metastability of the surfaces of catalysts, alongside how to control them to stabilize the dynamical active state, holds important implications for the future of catalysis.
In particular, it provides insights that can be transferred to the industrial level and the design of reactors where an active state with minimal energetic compromises is favored. This could be achieved either by using more potent oxidants, such as water (H 2 O) and nitrous oxide (N 2 O), or by working on reducing the oxygen leakage into the bulk by means of nanoparticles or thin film technology. The development of catalysts based on tailor-made thin films is the focus of CatLab, a joint research platform between the FHI, Helmholtz Center of Berlin (HZB).
More information: Luis Sandoval-Diaz et al, Metastable nickel–oxygen species modulate rate oscillations during dry reforming of methane, Nature Catalysis (2024). DOI: 10. 1038/s41929-023-01090-4 Journal information: Nature Catalysis Provided by Max Planck Society.
From: phys
URL: https://phys.org/news/2024-01-pros-cons-oxygen-nickel-catalysts.html